
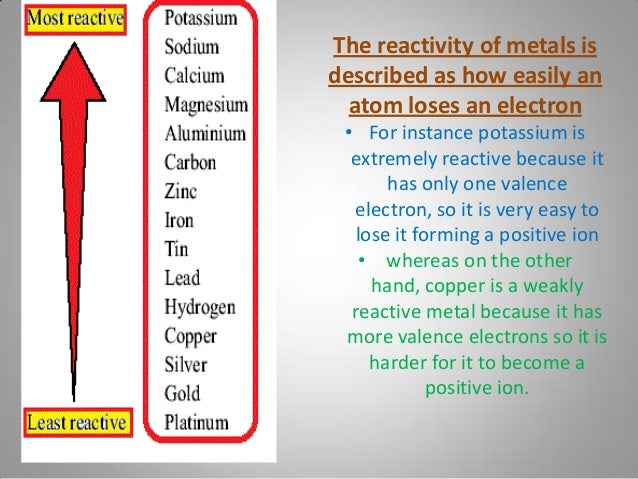
Just remember that the metal that displaces the other one is the more reactive one.Ĭlick here to go back to the Science menu. When displacement reaction equations is given, you should be able to put them in order of reactivity. Deduce an order of reactivity from a given set of experimental results.So overall, zinc finds it easier to lose its valence electrons than copper, and so is more reactive, and thus can displace copper. Zinc has more protons than copper but the other two conditions outweigh this one. Zinc is more reactive than copper because its valence electrons are farther away from the nucleus and it has more shells. there are fewer protons in the nucleus (less charge) to pull the electrons towards them.These shells shield the valence electrons from the charge of the nucleus (protons). there are more electron shells between the nucleus and the valence electrons.So it is easier to ‘let go’ of the electrons. When they are farther away, the attraction of these electrons (negative charge) to the protons (positive charge)in the nucleus will be reduced.

#Reactivity series chemistry gcse free#
The profit from every pack is reinvested into making free content on MME, which benefits millions of learners across the country. The MME Chemistry cards cover all the major topics areas within the AQA GCSE Chemistry specification. Reaction of metals and hydrogen with dilute (hydrochloric) acid: GCSE Chemistry revision cards are the perfect revision tool to help You improve your grade. Reaction of metals and hydrogen with water or steam: We can put metals in order of their reactivity by investigating how well they react with water or hydrochloric acid. dilute hydrochloric acid (except for alkali metals).Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, hydrogen and copper, by reference to the reactions, if any, of the elements with:.We apologise for the inconvenience, but hope that the new images will provide you with an even better learning experience. Sulfate ions, SO 4 2-, appear on both sides of the equation because they do not take part in the reaction.Disclaimer: Due to unforeseen difficulties, we have had to take down the images on this notes page. The equation below shows the ions present on both sides of the equation: Here is the balanced ionic equation for the reaction between magnesium and copper(II) sulfate. Magnesium is the most reactive metal from these three as it undergoes two reactions, iron is next and copper is the least reactive.ĭisplacement reactions as redox reactions The table below shows the results of reacting different metals with different salt solutions. You can work out a reactivity series for many substances by carrying out displacement reactions.


 0 kommentar(er)
0 kommentar(er)
